Annotate single cells using SingleR
Usage
RunSingleR(
srt_query,
srt_ref,
query_group = NULL,
ref_group = NULL,
query_assay = "RNA",
ref_assay = "RNA",
genes = "de",
de.method = "wilcox",
sd.thresh = 1,
de.n = NULL,
aggr.ref = FALSE,
aggr.args = list(),
quantile = 0.8,
fine.tune = TRUE,
tune.thresh = 0.05,
prune = TRUE,
cores = 1,
verbose = TRUE
)Arguments
- srt_query
An object of class Seurat to be annotated with cell types.
- srt_ref
An object of class Seurat storing the reference cells.
- query_group
A character vector specifying the column name in the
srt_querymetadata that represents the cell grouping.- ref_group
A character vector specifying the column name in the
srt_refmetadata that represents the cell grouping.- query_assay
A character vector specifying the assay to be used for the query data. Default is the default assay of the
srt_queryobject.- ref_assay
A character vector specifying the assay to be used for the reference data. Default is the default assay of the
srt_refobject.- genes
"genes"parameter in SingleR::SingleR function.- de.method
"de.method"parameter in SingleR::SingleR function.- sd.thresh
Deprecated and ignored.
- de.n
An integer scalar specifying the number of DE genes to use when
genes="de". Ifde.method="classic", defaults to500 * (2/3) ^ log2(N)whereNis the number of unique labels. Otherwise, defaults to 10. Ignored ifgenesis a list of markers/DE genes.- aggr.ref, aggr.args
Arguments controlling the aggregation of the references prior to annotation, see
trainSingleR.- quantile
"quantile" parameter in SingleR::SingleR function.
- fine.tune
"fine.tune"parameter in SingleR::SingleR function.- tune.thresh
"tune.thresh"parameter in SingleR::SingleR function.- prune
"prune"parameter in SingleR::SingleR function.- cores
The number of cores to use for parallelization with foreach::foreach. Default is
1.- verbose
Whether to print the message. Default is
TRUE.
Examples
data(panc8_sub)
# Simply convert genes from human to mouse and preprocess the data
genenames <- make.unique(
thisutils::capitalize(
rownames(panc8_sub),
force_tolower = TRUE
)
)
names(genenames) <- rownames(panc8_sub)
panc8_sub <- RenameFeatures(
panc8_sub,
newnames = genenames
)
#> ℹ [2026-02-11 04:10:25] Rename features for the assay: RNA
panc8_sub <- CheckDataMerge(
panc8_sub,
batch = "tech"
)[["srt_merge"]]
#> ℹ [2026-02-11 04:10:25] Spliting `srt_merge` into `srt_list` by column "tech"...
#> ℹ [2026-02-11 04:10:26] Checking a list of <Seurat>...
#> ! [2026-02-11 04:10:26] Data 1/5 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 04:10:26] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 1/5 of the `srt_list`...
#> ℹ [2026-02-11 04:10:28] Perform `Seurat::FindVariableFeatures()` on the data 1/5 of the `srt_list`...
#> ! [2026-02-11 04:10:28] Data 2/5 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 04:10:28] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 2/5 of the `srt_list`...
#> ℹ [2026-02-11 04:10:30] Perform `Seurat::FindVariableFeatures()` on the data 2/5 of the `srt_list`...
#> ! [2026-02-11 04:10:30] Data 3/5 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 04:10:30] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 3/5 of the `srt_list`...
#> ℹ [2026-02-11 04:10:32] Perform `Seurat::FindVariableFeatures()` on the data 3/5 of the `srt_list`...
#> ! [2026-02-11 04:10:32] Data 4/5 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 04:10:32] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 4/5 of the `srt_list`...
#> ℹ [2026-02-11 04:10:34] Perform `Seurat::FindVariableFeatures()` on the data 4/5 of the `srt_list`...
#> ! [2026-02-11 04:10:34] Data 5/5 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 04:10:34] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 5/5 of the `srt_list`...
#> ℹ [2026-02-11 04:10:36] Perform `Seurat::FindVariableFeatures()` on the data 5/5 of the `srt_list`...
#> ℹ [2026-02-11 04:10:36] Use the separate HVF from srt_list
#> ℹ [2026-02-11 04:10:37] Number of available HVF: 2000
#> ℹ [2026-02-11 04:10:37] Finished check
# Annotation
data(pancreas_sub)
pancreas_sub <- standard_scop(pancreas_sub)
#> ℹ [2026-02-11 04:10:40] Start standard scop workflow...
#> ℹ [2026-02-11 04:10:41] Checking a list of <Seurat>...
#> ! [2026-02-11 04:10:41] Data 1/1 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 04:10:41] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 1/1 of the `srt_list`...
#> ℹ [2026-02-11 04:10:43] Perform `Seurat::FindVariableFeatures()` on the data 1/1 of the `srt_list`...
#> ℹ [2026-02-11 04:10:43] Use the separate HVF from srt_list
#> ℹ [2026-02-11 04:10:43] Number of available HVF: 2000
#> ℹ [2026-02-11 04:10:43] Finished check
#> ℹ [2026-02-11 04:10:44] Perform `Seurat::ScaleData()`
#> ℹ [2026-02-11 04:10:44] Perform pca linear dimension reduction
#> ℹ [2026-02-11 04:10:45] Perform `Seurat::FindClusters()` with `cluster_algorithm = 'louvain'` and `cluster_resolution = 0.6`
#> ℹ [2026-02-11 04:10:45] Reorder clusters...
#> ℹ [2026-02-11 04:10:45] Perform umap nonlinear dimension reduction
#> ℹ [2026-02-11 04:10:45] Non-linear dimensionality reduction (umap) using (Standardpca) dims (1-50) as input
#> ℹ [2026-02-11 04:10:50] Non-linear dimensionality reduction (umap) using (Standardpca) dims (1-50) as input
#> ✔ [2026-02-11 04:10:54] Run scop standard workflow completed
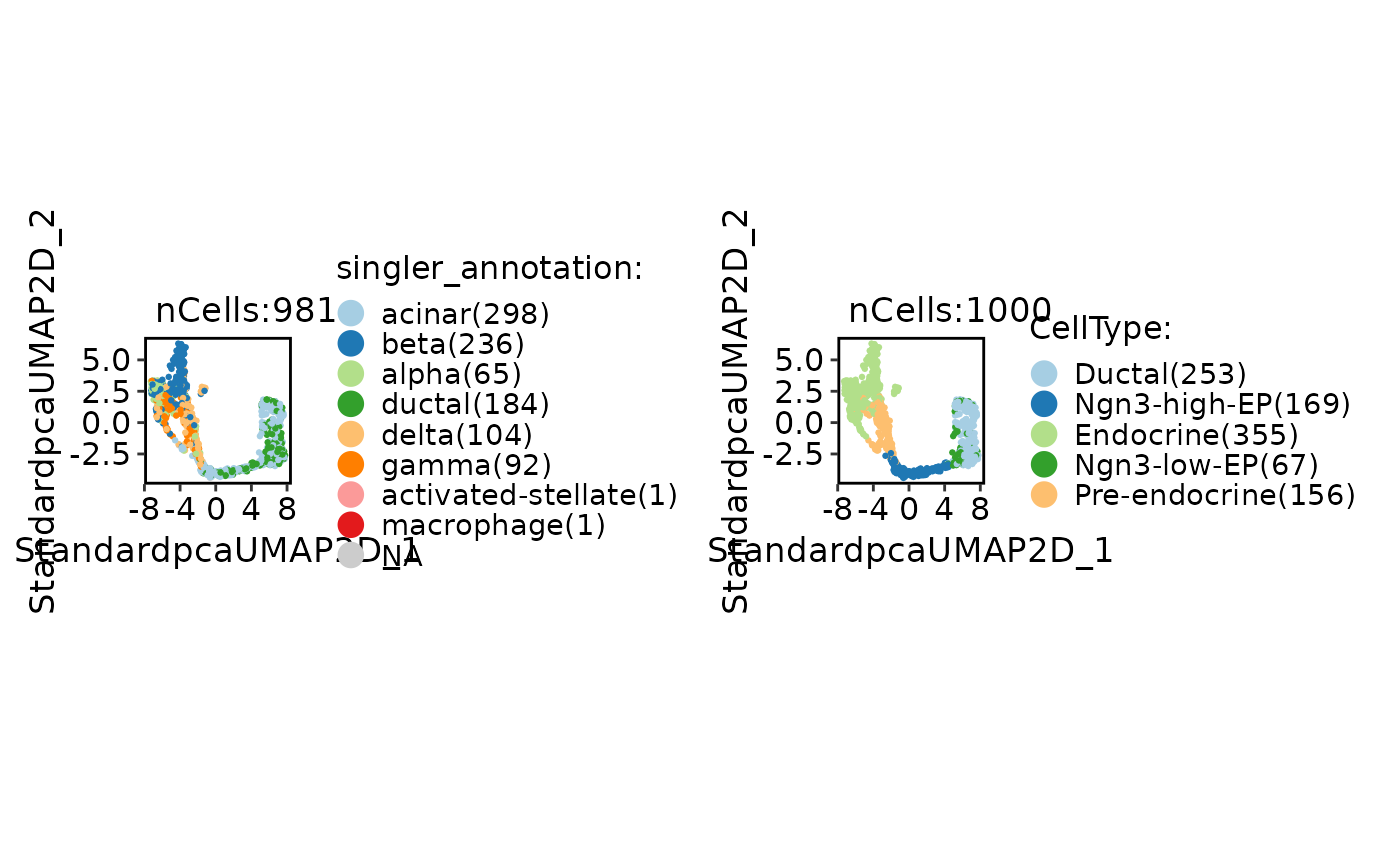
pancreas_sub <- RunSingleR(
srt_query = pancreas_sub,
srt_ref = panc8_sub,
query_group = "Standardpca_SNN_res.0.6",
ref_group = "celltype"
)
#> ℹ [2026-02-11 04:10:54] Start SingleR annotation
#> ℹ [2026-02-11 04:17:37] Data type is log-normalized
#> ℹ [2026-02-11 04:17:37] Detected `srt_query` data type: "log_normalized_counts"
#> ℹ [2026-02-11 04:17:39] Data type is log-normalized
#> ℹ [2026-02-11 04:17:39] Detected `srt_ref` data type: "log_normalized_counts"
#> ℹ [2026-02-11 04:17:43] Perform "SingleRCluster"
#> ✔ [2026-02-11 04:17:44] SingleR annotation completed
CellDimPlot(
pancreas_sub,
group.by = c("singler_annotation", "CellType")
)
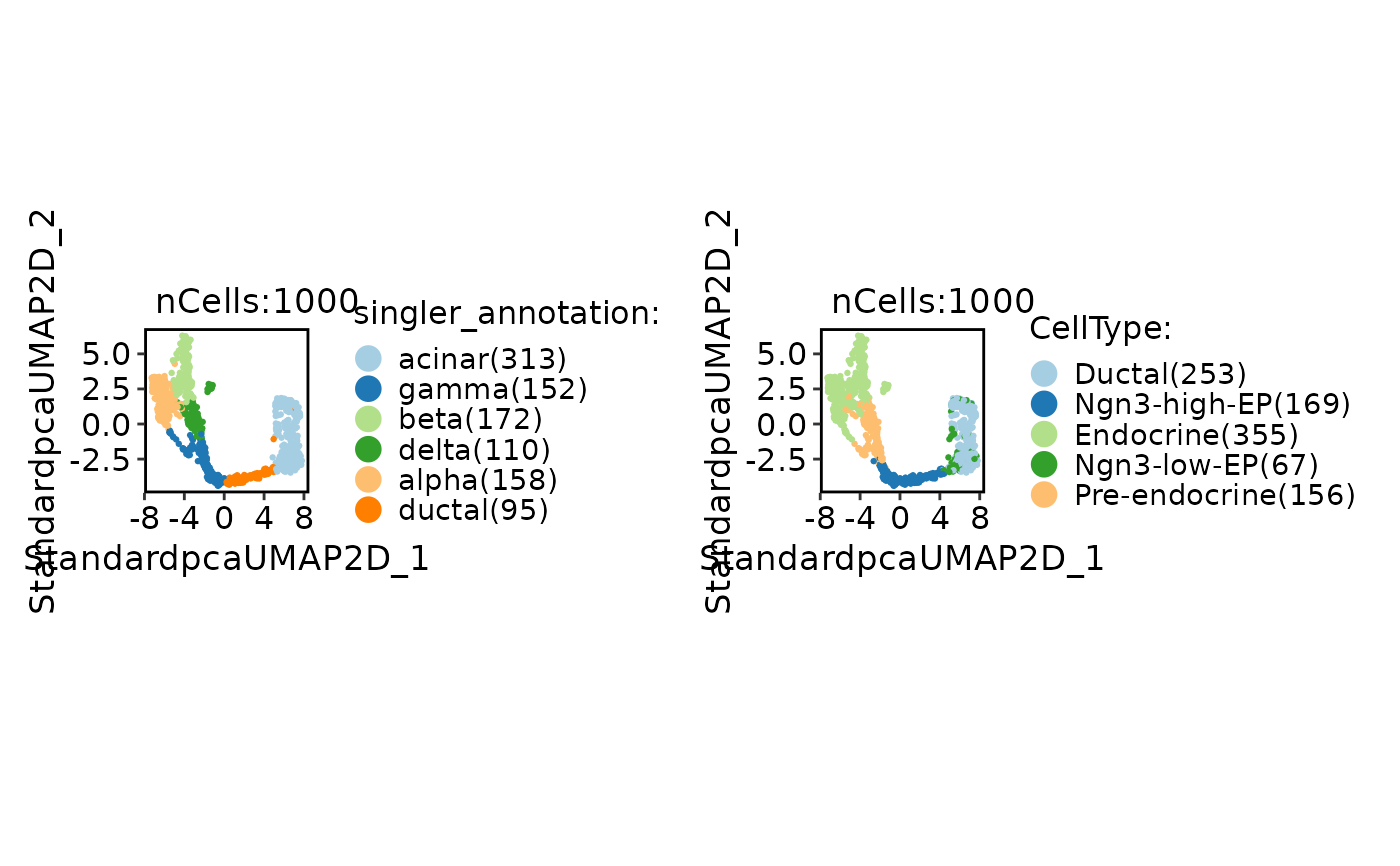 pancreas_sub <- RunSingleR(
srt_query = pancreas_sub,
srt_ref = panc8_sub,
query_group = NULL,
ref_group = "celltype"
)
#> ℹ [2026-02-11 04:17:45] Start SingleR annotation
#> ℹ [2026-02-11 04:17:45] Data type is log-normalized
#> ℹ [2026-02-11 04:17:45] Detected `srt_query` data type: "log_normalized_counts"
#> ℹ [2026-02-11 04:17:48] Data type is log-normalized
#> ℹ [2026-02-11 04:17:48] Detected `srt_ref` data type: "log_normalized_counts"
#> ℹ [2026-02-11 04:17:54] Perform "SingleRCell"
#> ✔ [2026-02-11 04:17:58] SingleR annotation completed
CellDimPlot(
pancreas_sub,
group.by = c("singler_annotation", "CellType")
)
pancreas_sub <- RunSingleR(
srt_query = pancreas_sub,
srt_ref = panc8_sub,
query_group = NULL,
ref_group = "celltype"
)
#> ℹ [2026-02-11 04:17:45] Start SingleR annotation
#> ℹ [2026-02-11 04:17:45] Data type is log-normalized
#> ℹ [2026-02-11 04:17:45] Detected `srt_query` data type: "log_normalized_counts"
#> ℹ [2026-02-11 04:17:48] Data type is log-normalized
#> ℹ [2026-02-11 04:17:48] Detected `srt_ref` data type: "log_normalized_counts"
#> ℹ [2026-02-11 04:17:54] Perform "SingleRCell"
#> ✔ [2026-02-11 04:17:58] SingleR annotation completed
CellDimPlot(
pancreas_sub,
group.by = c("singler_annotation", "CellType")
)