This function creates a correlation plot to visualize the pairwise correlations between selected features in a Seurat object.
Usage
FeatureCorPlot(
srt,
features,
group.by = NULL,
split.by = NULL,
cells = NULL,
layer = "data",
assay = NULL,
cor_method = "pearson",
adjust = 1,
margin = 1,
reverse = FALSE,
add_equation = FALSE,
add_r2 = TRUE,
add_pvalue = TRUE,
add_smooth = TRUE,
palette = "Paired",
palcolor = NULL,
cor_palette = "RdBu",
cor_palcolor = NULL,
cor_range = c(-1, 1),
pt.size = NULL,
pt.alpha = 1,
cells.highlight = NULL,
cols.highlight = "black",
sizes.highlight = 1,
alpha.highlight = 1,
stroke.highlight = 0.5,
calculate_coexp = FALSE,
raster = NULL,
raster.dpi = c(512, 512),
aspect.ratio = 1,
title = NULL,
subtitle = NULL,
legend.position = "right",
legend.direction = "vertical",
theme_use = "theme_scop",
theme_args = list(),
combine = TRUE,
nrow = NULL,
ncol = NULL,
byrow = TRUE,
force = FALSE,
seed = 11
)Arguments
- srt
A Seurat object.
- features
A character vector specifying the features to compare. Should be present in both the assay data and the metadata of the Seurat object.
- group.by
Name of one or more meta.data columns to group (color) cells by.
- split.by
Name of a column in meta.data column to split plot by. Default is
NULL.- cells
A character vector of cell names to use.
- layer
Which layer to use. Default is
data.- assay
Which assay to use. If
NULL, the default assay of the Seurat object will be used.- cor_method
A character string specifying the correlation method to use. Can be
"pearson"or"spearman". Default is"pearson".- adjust
The adjustment factor for the width of the violin plots. Default is
1.- margin
The margin size for the plot. Default is
1.- reverse
Whether to reverse the order of the features in the plot. Default is
FALSE.- add_equation
Whether to add the equation of the linear regression line to each scatter plot. Default is
FALSE.- add_r2
Whether to add the R-squared value of the linear regression line to each scatter plot. Default is
TRUE.- add_pvalue
Whether to add the p-value of the linear regression line to each scatter plot. Default is
TRUE.- add_smooth
Whether to add a smoothed line to each scatter plot. Default is
TRUE.- palette
Color palette name. Available palettes can be found in thisplot::show_palettes. Default is
"Paired".- palcolor
Custom colors used to create a color palette. Default is
NULL.- cor_palette
A character string specifying the name of the color palette to use for the correlation. Default is
"RdBu".- cor_palcolor
A character string specifying the color for the correlation. Default is
"RdBu".- cor_range
A two-length numeric vector specifying the range for the correlation.
- pt.size
The size of the points in the plot.
- pt.alpha
The transparency of the data points. Default is
1.- cells.highlight
A logical or character vector specifying the cells to highlight in the plot. If
TRUE, all cells are highlighted. IfFALSE, no cells are highlighted. Default isNULL.- cols.highlight
Color used to highlight the cells.
- sizes.highlight
Size of highlighted cell points.
- alpha.highlight
Transparency of highlighted cell points.
- stroke.highlight
Border width of highlighted cell points.
- calculate_coexp
Whether to calculate the co-expression of selected features. Default is
FALSE.- raster
Convert points to raster format. Default is
NULL, which automatically rasterizes if plotting more than 100,000 cells.- raster.dpi
Pixel resolution for rasterized plots. Default is
c(512, 512).- aspect.ratio
Aspect ratio of the panel. Default is
1.- title
The text for the title. Default is
NULL.- subtitle
The text for the subtitle for the plot which will be displayed below the title. Default is
NULL.- legend.position
The position of legends, one of
"none","left","right","bottom","top". Default is"right".- legend.direction
The direction of the legend in the plot. Can be one of
"vertical"or"horizontal".- theme_use
Theme used. Can be a character string or a theme function. Default is
"theme_scop".- theme_args
Other arguments passed to the
theme_use. Default islist().- combine
Combine plots into a single
patchworkobject. IfFALSE, return a list of ggplot objects.- nrow
Number of rows in the combined plot. Default is
NULL, which means determined automatically based on the number of plots.- ncol
Number of columns in the combined plot. Default is
NULL, which means determined automatically based on the number of plots.- byrow
Whether to arrange the plots by row in the combined plot. Default is
TRUE.- force
Whether to force the creation of the plot, even if it contains more than 50 subplots. Default is
FALSE.- seed
Random seed for reproducibility. Default is
11.
Examples
data(pancreas_sub)
pancreas_sub <- standard_scop(pancreas_sub)
#> ℹ [2026-02-11 03:31:14] Start standard scop workflow...
#> ℹ [2026-02-11 03:31:14] Checking a list of <Seurat>...
#> ! [2026-02-11 03:31:14] Data 1/1 of the `srt_list` is "unknown"
#> ℹ [2026-02-11 03:31:14] Perform `NormalizeData()` with `normalization.method = 'LogNormalize'` on the data 1/1 of the `srt_list`...
#> ℹ [2026-02-11 03:31:16] Perform `Seurat::FindVariableFeatures()` on the data 1/1 of the `srt_list`...
#> ℹ [2026-02-11 03:31:17] Use the separate HVF from srt_list
#> ℹ [2026-02-11 03:31:17] Number of available HVF: 2000
#> ℹ [2026-02-11 03:31:17] Finished check
#> ℹ [2026-02-11 03:31:17] Perform `Seurat::ScaleData()`
#> ℹ [2026-02-11 03:31:18] Perform pca linear dimension reduction
#> ℹ [2026-02-11 03:31:18] Perform `Seurat::FindClusters()` with `cluster_algorithm = 'louvain'` and `cluster_resolution = 0.6`
#> ℹ [2026-02-11 03:31:19] Reorder clusters...
#> ℹ [2026-02-11 03:31:19] Perform umap nonlinear dimension reduction
#> ℹ [2026-02-11 03:31:19] Non-linear dimensionality reduction (umap) using (Standardpca) dims (1-50) as input
#> ℹ [2026-02-11 03:31:22] Non-linear dimensionality reduction (umap) using (Standardpca) dims (1-50) as input
#> ✔ [2026-02-11 03:31:25] Run scop standard workflow completed
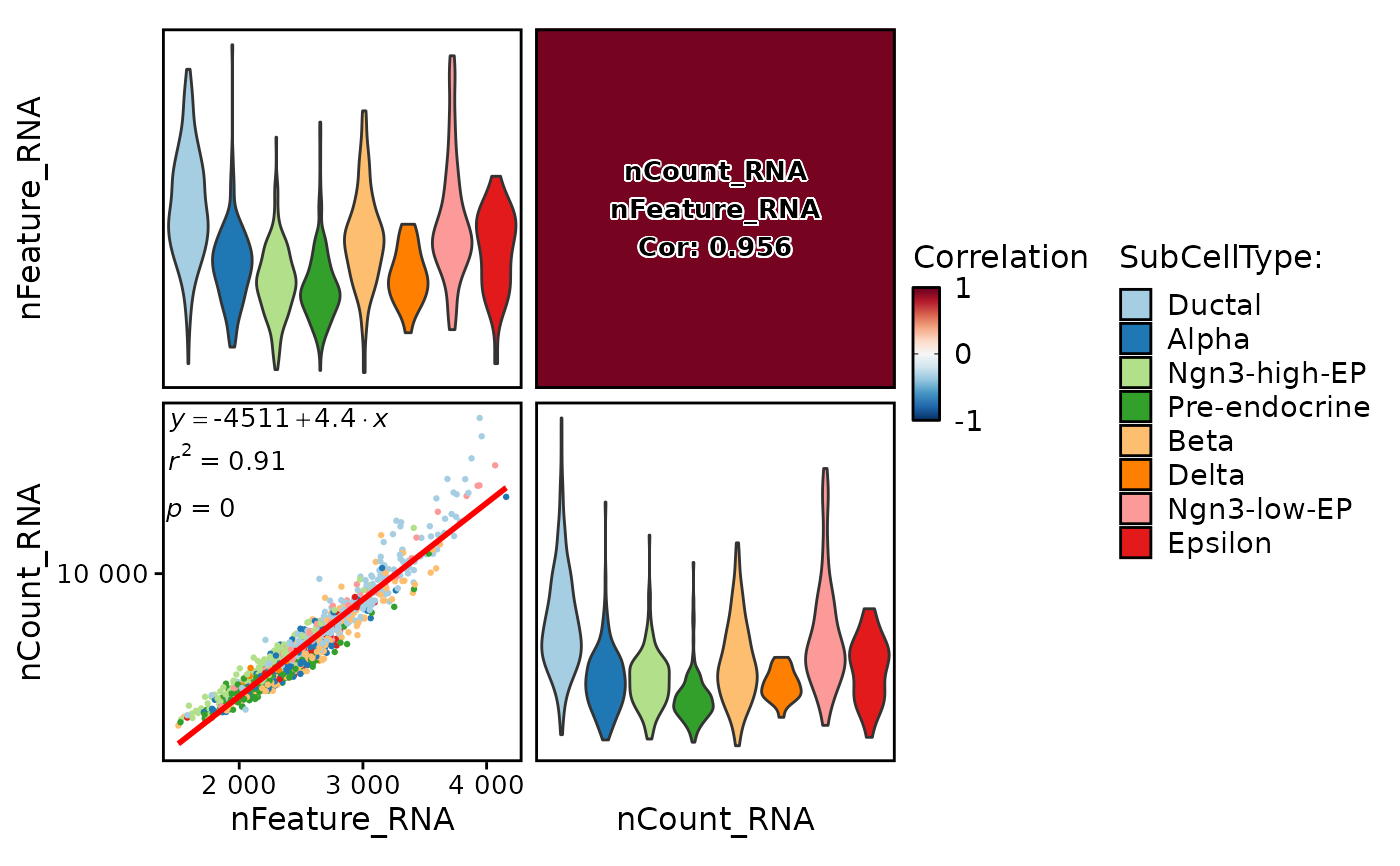
FeatureCorPlot(
pancreas_sub,
features = rownames(pancreas_sub)[1:5],
group.by = "SubCellType"
)
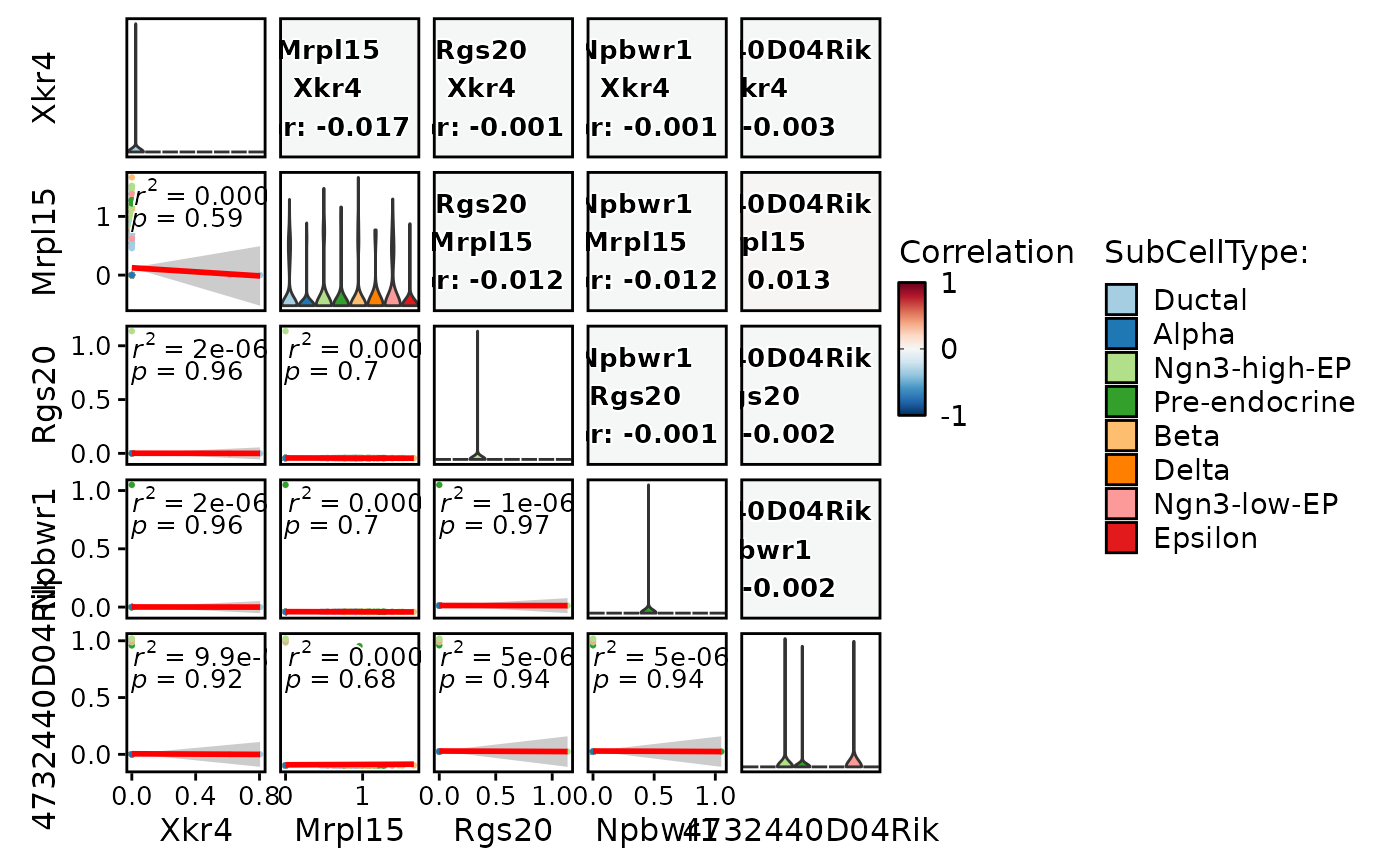 FeatureCorPlot(
pancreas_sub,
features = c(
"nFeature_RNA",
"nCount_RNA",
"nFeature_spliced",
"nCount_spliced",
"nFeature_unspliced",
"nCount_unspliced"
),
group.by = "SubCellType",
cor_palette = "Greys",
cor_range = c(0, 1)
)
#> Warning: essentially perfect fit: summary may be unreliable
#> Warning: essentially perfect fit: summary may be unreliable
#> Warning: essentially perfect fit: summary may be unreliable
#> Warning: essentially perfect fit: summary may be unreliable
FeatureCorPlot(
pancreas_sub,
features = c(
"nFeature_RNA",
"nCount_RNA",
"nFeature_spliced",
"nCount_spliced",
"nFeature_unspliced",
"nCount_unspliced"
),
group.by = "SubCellType",
cor_palette = "Greys",
cor_range = c(0, 1)
)
#> Warning: essentially perfect fit: summary may be unreliable
#> Warning: essentially perfect fit: summary may be unreliable
#> Warning: essentially perfect fit: summary may be unreliable
#> Warning: essentially perfect fit: summary may be unreliable
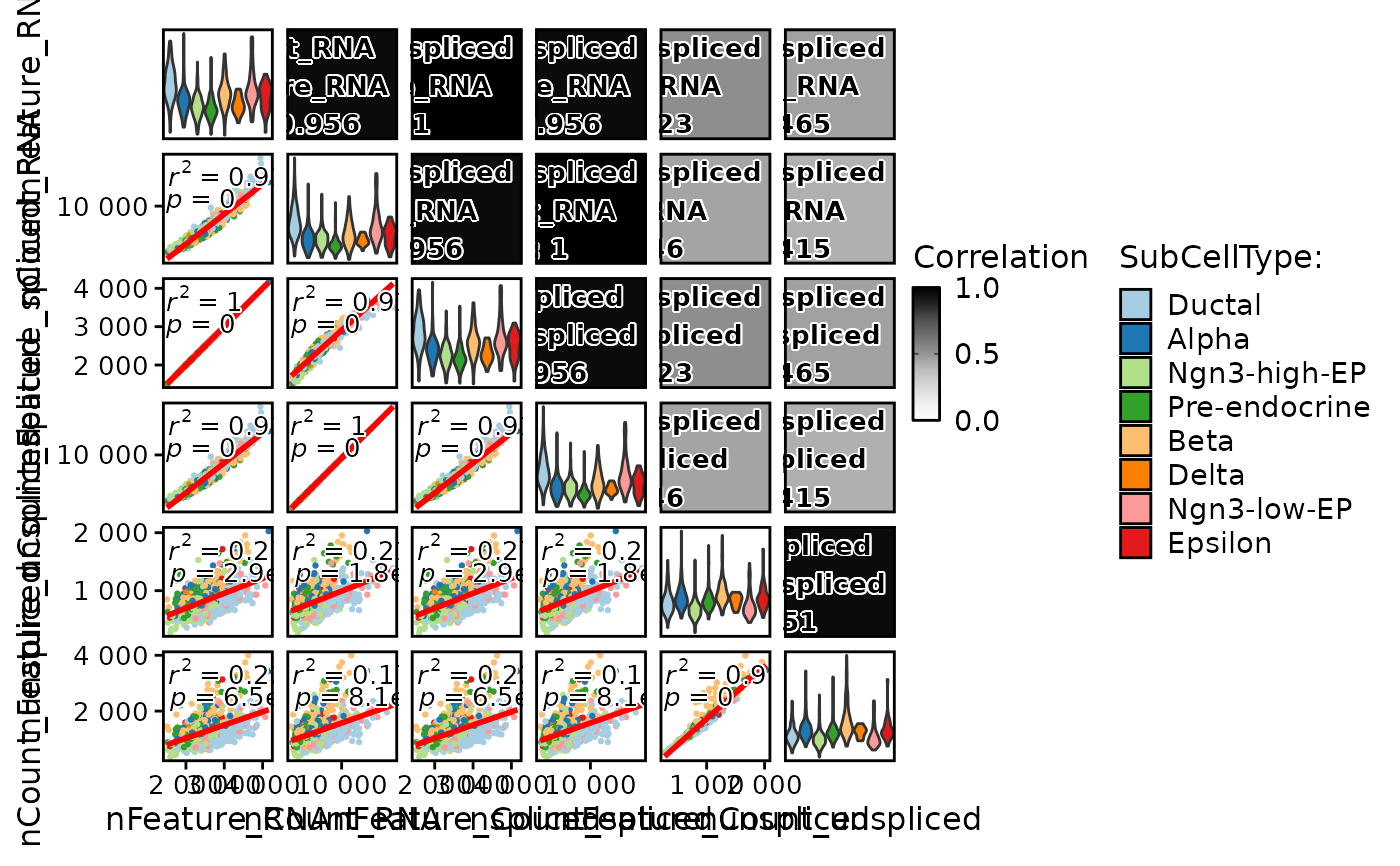 FeatureCorPlot(
pancreas_sub,
features = c("nFeature_RNA", "nCount_RNA"),
group.by = "SubCellType",
add_equation = TRUE
)
FeatureCorPlot(
pancreas_sub,
features = c("nFeature_RNA", "nCount_RNA"),
group.by = "SubCellType",
add_equation = TRUE
)